Like your gardener says Run North man Run North!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Stop in for a cup of coffee
- Thread starter toolmanmike
- Start date
-
Man people are seriously unlading stuff n flea bay. Almost every Item I saved has gotten discount offers.
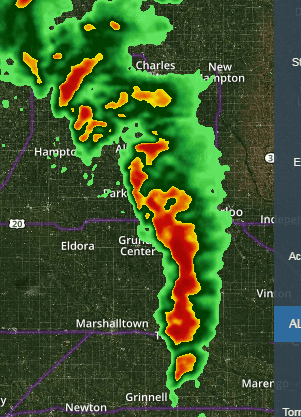
And the thunder rolls. It must be a pretty good storm. Constant thunder. Yep, just about here.
zkx14
Duster De-ruster
Been jonesin for this since you posted. Picked up a 4 pack this morning before work and did decon.Just another redneck day working on the Demon! Glad to be back home and slaving out in the shop. Pandora’s Whiskey Meyers on for jams.
View attachment 1715495783
View attachment 1715495784
Chris brought over some home made Limoncello. Damn the stuff is good.

Ccw?Firing order 18436572. CCW.
Opposite the way a turd flushes!
Raining pitchforks and hammer handles. Small hail.
Unconventional
Well-Known Member
Unconventional
Well-Known Member
Raining pitchforks and hammer handles. Small hail.
I was just lookin' at the Ark-Tenn-Miss border area. They got some serious goin's on there too.
See a picture is worth a thousand turds!
Ddaddy
Just doing what I do
More good news in the virus fight today...
New Corona Virus test in less than 5 minutes and is portable to go anywhere
Abbott Laboratories is unveiling a coronavirus test that can tell if someone is infected in as little as five minutes, and is so small and portable it can be used in almost any health-care setting.
The medical-device maker plans to supply 50,000 tests a day starting April 1, said John Frels, vice president of research and development at Abbott Diagnostics. The molecular test looks for fragments of the coronavirus genome, which can quickly be detected when present at high levels. A thorough search to definitively rule out an infection can take up to 13 minutes, he said.
Abbott has received emergency use authorization from the U.S. Food and Drug Administration “for use by authorized laboratories and patient care settings,” the company said on Friday.
The test starts with taking a swab from the nose or the back of the throat, then mixing it with a chemical solution that breaks open the virus and releases its RNA. The mixture is inserted into an ID Now system, a small box weighing just under 7 pounds that has the technology to identify and amplify select sequences of the coronavirus genome and ignore contamination from other viruses.
The equipment can be set up almost anywhere, but the company is working with its customers and the Trump administration to ensure the first cartridges used to perform the tests are sent to where they are most needed. They are targeting hospital emergency rooms, urgent-care clinics and doctors’ offices.
Last week, Abbott’s m2000 RealTime system got U.S. Food and Drug Administration approval for use in hospitals and molecular laboratories to diagnose the infection. That system can churn through more tests on a daily basis, up to 1 million a week, but it takes longer to get the results. Abbott plans to provide at least 5 million tests a month between the two systems.
Other companies are also rolling out faster testing systems. Henry Schein Inc. on Thursday said its point-of-care antibody test, which looks for evidence that a person’s immune system has already fought off the infection, was available. The blood test can be given at the point of care and delivers results in about 15 minutes, though it can’t be used to definitively diagnose a current infection.
New Corona Virus test in less than 5 minutes and is portable to go anywhere
Abbott Laboratories is unveiling a coronavirus test that can tell if someone is infected in as little as five minutes, and is so small and portable it can be used in almost any health-care setting.
The medical-device maker plans to supply 50,000 tests a day starting April 1, said John Frels, vice president of research and development at Abbott Diagnostics. The molecular test looks for fragments of the coronavirus genome, which can quickly be detected when present at high levels. A thorough search to definitively rule out an infection can take up to 13 minutes, he said.
Abbott has received emergency use authorization from the U.S. Food and Drug Administration “for use by authorized laboratories and patient care settings,” the company said on Friday.
The test starts with taking a swab from the nose or the back of the throat, then mixing it with a chemical solution that breaks open the virus and releases its RNA. The mixture is inserted into an ID Now system, a small box weighing just under 7 pounds that has the technology to identify and amplify select sequences of the coronavirus genome and ignore contamination from other viruses.
The equipment can be set up almost anywhere, but the company is working with its customers and the Trump administration to ensure the first cartridges used to perform the tests are sent to where they are most needed. They are targeting hospital emergency rooms, urgent-care clinics and doctors’ offices.
Last week, Abbott’s m2000 RealTime system got U.S. Food and Drug Administration approval for use in hospitals and molecular laboratories to diagnose the infection. That system can churn through more tests on a daily basis, up to 1 million a week, but it takes longer to get the results. Abbott plans to provide at least 5 million tests a month between the two systems.
Other companies are also rolling out faster testing systems. Henry Schein Inc. on Thursday said its point-of-care antibody test, which looks for evidence that a person’s immune system has already fought off the infection, was available. The blood test can be given at the point of care and delivers results in about 15 minutes, though it can’t be used to definitively diagnose a current infection.
Tornado warning. We just go outside and watch.
LOL. Clock wise!!! I have been working on 383. Got confused!!!!Ccw?
Ddaddy
Just doing what I do
You are still confused. CCW is counter-clock wise!LOL. Clock wise!!! I have been working on 383. Got confused!!!!
dukeboy440
Well-Known Member
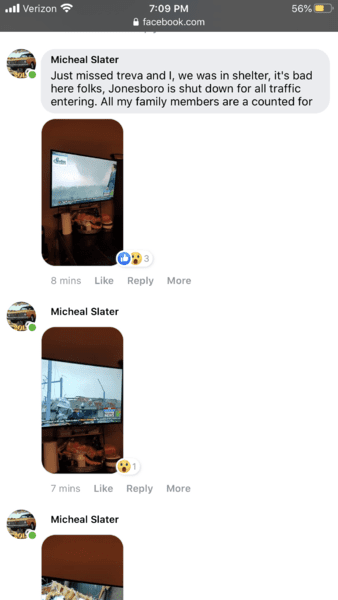
Yeah me mike said he was in the path. Haven’t heard an update from him yet. Hope he’s okI was just lookin' at the Ark-Tenn-Miss border area. They got some serious goin's on there too.
Unconventional
Well-Known Member
More good news in the virus fight today...
New Corona Virus test in less than 5 minutes and is portable to go anywhere
Abbott Laboratories is unveiling a coronavirus test that can tell if someone is infected in as little as five minutes, and is so small and portable it can be used in almost any health-care setting.
The medical-device maker plans to supply 50,000 tests a day starting April 1, said John Frels, vice president of research and development at Abbott Diagnostics. The molecular test looks for fragments of the coronavirus genome, which can quickly be detected when present at high levels. A thorough search to definitively rule out an infection can take up to 13 minutes, he said.
Abbott has received emergency use authorization from the U.S. Food and Drug Administration “for use by authorized laboratories and patient care settings,” the company said on Friday.
The test starts with taking a swab from the nose or the back of the throat, then mixing it with a chemical solution that breaks open the virus and releases its RNA. The mixture is inserted into an ID Now system, a small box weighing just under 7 pounds that has the technology to identify and amplify select sequences of the coronavirus genome and ignore contamination from other viruses.
The equipment can be set up almost anywhere, but the company is working with its customers and the Trump administration to ensure the first cartridges used to perform the tests are sent to where they are most needed. They are targeting hospital emergency rooms, urgent-care clinics and doctors’ offices.
Last week, Abbott’s m2000 RealTime system got U.S. Food and Drug Administration approval for use in hospitals and molecular laboratories to diagnose the infection. That system can churn through more tests on a daily basis, up to 1 million a week, but it takes longer to get the results. Abbott plans to provide at least 5 million tests a month between the two systems.
Other companies are also rolling out faster testing systems. Henry Schein Inc. on Thursday said its point-of-care antibody test, which looks for evidence that a person’s immune system has already fought off the infection, was available. The blood test can be given at the point of care and delivers results in about 15 minutes, though it can’t be used to definitively diagnose a current infection.
Amazing
Sparkies 318You are still confused. CCW is counter-clock wise!
Ddaddy
Just doing what I do
Those of us in the Pharmaceutical industry aren’t sitting back waiting to be instructed what to do. You would be amazed at the number of fast moving initiatives that are out there across the industry. We just need everyone to help flatten the curve and buy us time.Amazing
We can defeat this thing, we have the brain power and determination...we just need time.
Any stock tips?
























318 is a clockwise rotation, I posted counter clockwise...You are still confused. CCW is counter-clock wise!
dukeboy440
Well-Known Member
Looks like it missed him
dukeboy440
Well-Known Member
I know, I’ll let it slide, this once haha318 is a clockwise rotation, I posted counter clockwise...
Ddaddy
Just doing what I do
If it’s for Chris, it won’t matter which way it rotates...it isn’t going to start anytime soon. And I guarantee if and when it does, it will break something quickly.318 is a clockwise rotation, I posted counter clockwise...
zkx14
Duster De-ruster
Too many people in work tonight. Only a handful of presses running. But,Usually the one department works short shifts on Saturday and are gone by 5PM. Most of them are here all night. Not a big deal for my work, but with current **** goin around I was feeling a bit crowded in the lunch area. So came back to the office to finish my break.
-

 Got it!
Got it!














